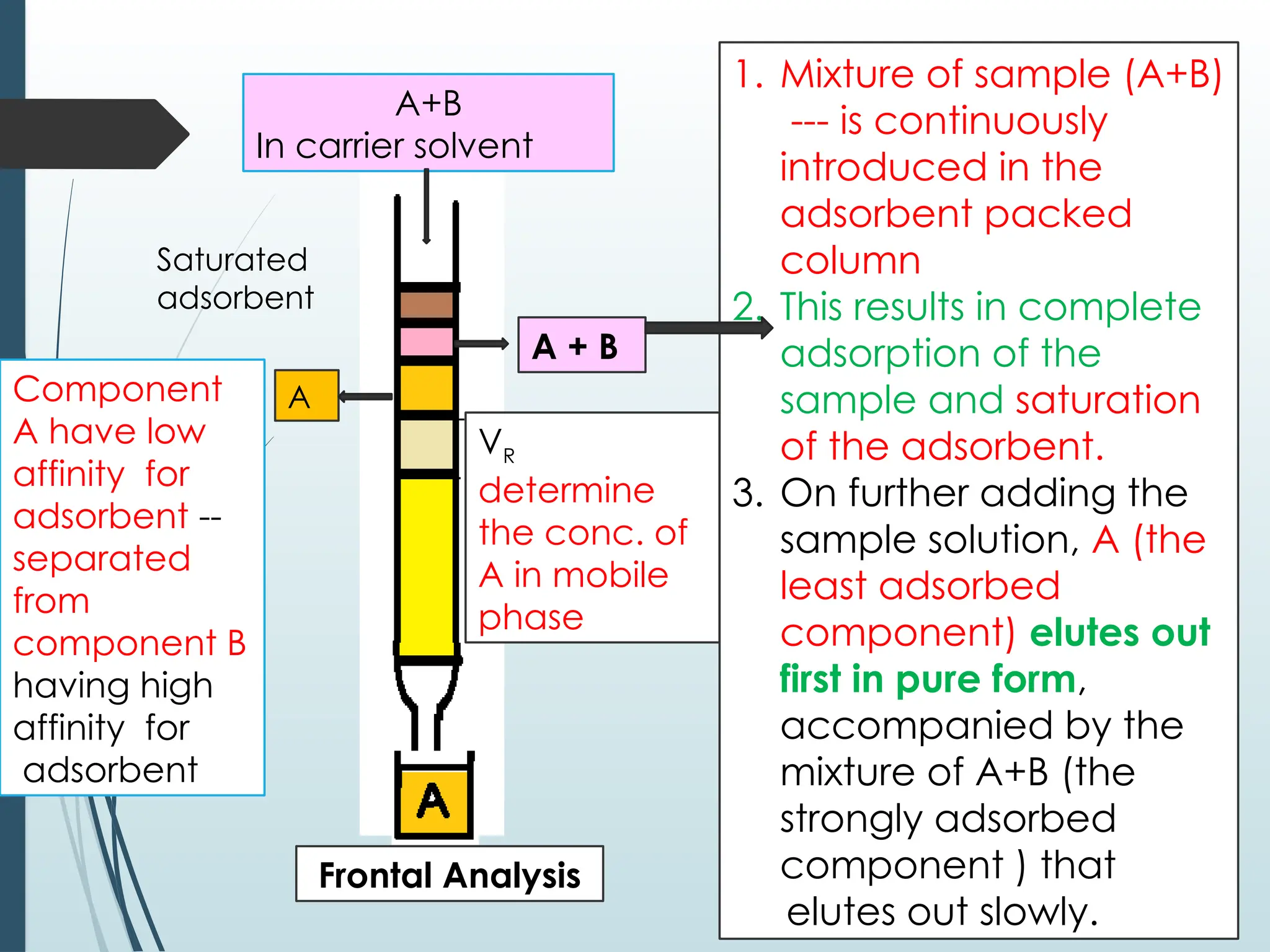
The document outlines the principles and methodologies of chromatography, detailing types such as partition, adsorption, and ion exchange chromatography. It explains the roles of stationary and mobile phases, factors affecting separation, and various chromatographic techniques used for analysis and purification. Additionally, it highlights the significance of concepts like partition coefficients and retention factors in chromatography.