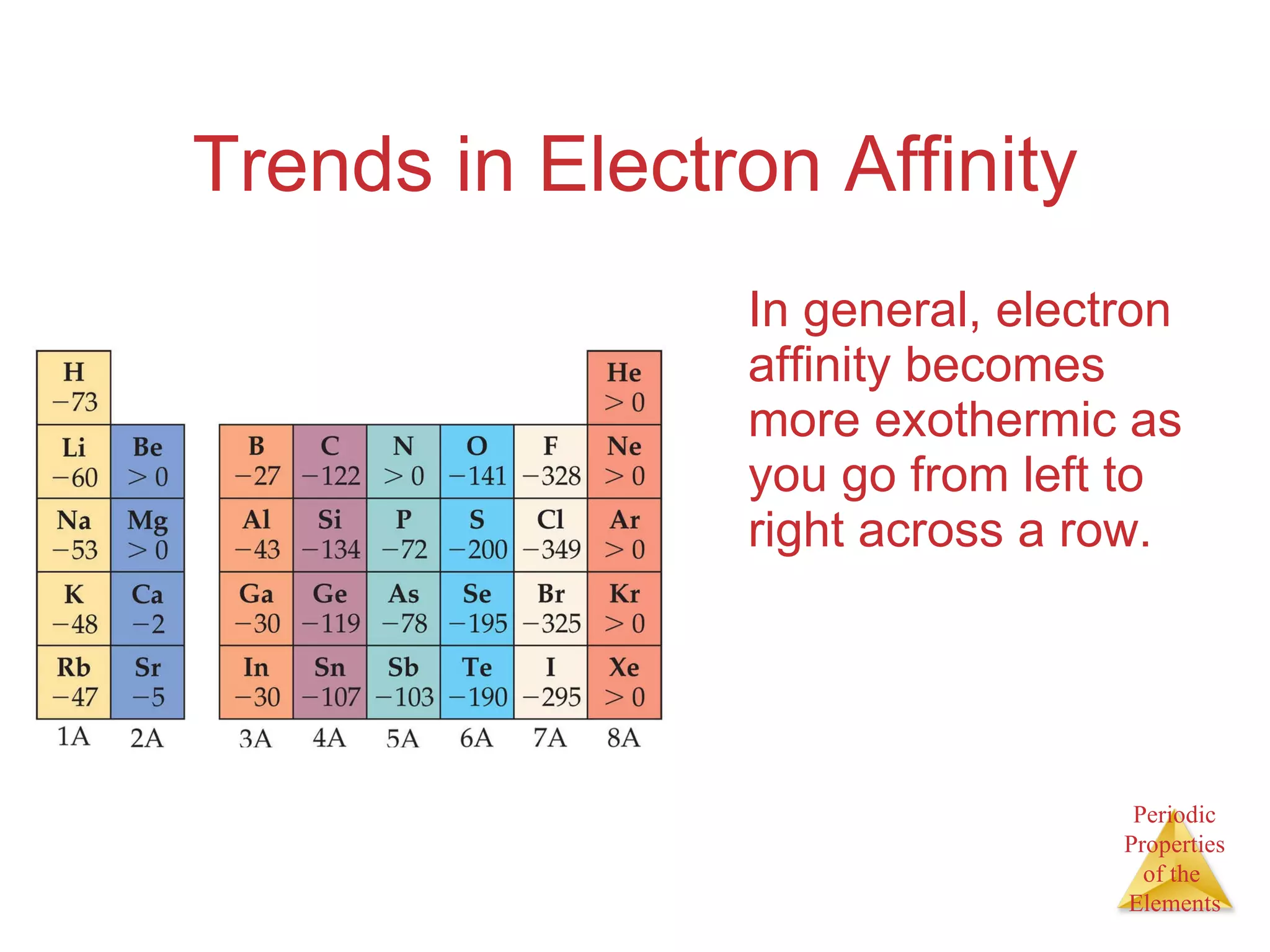
The document discusses trends in the properties of elements in the periodic table. It describes how elements in the same group generally have similar chemical properties, though properties are not identical. It discusses how Mendeleev and Meyer independently organized the elements into the first periodic tables based on recurring trends in properties. Elements in the inner transition metals block were later added.




































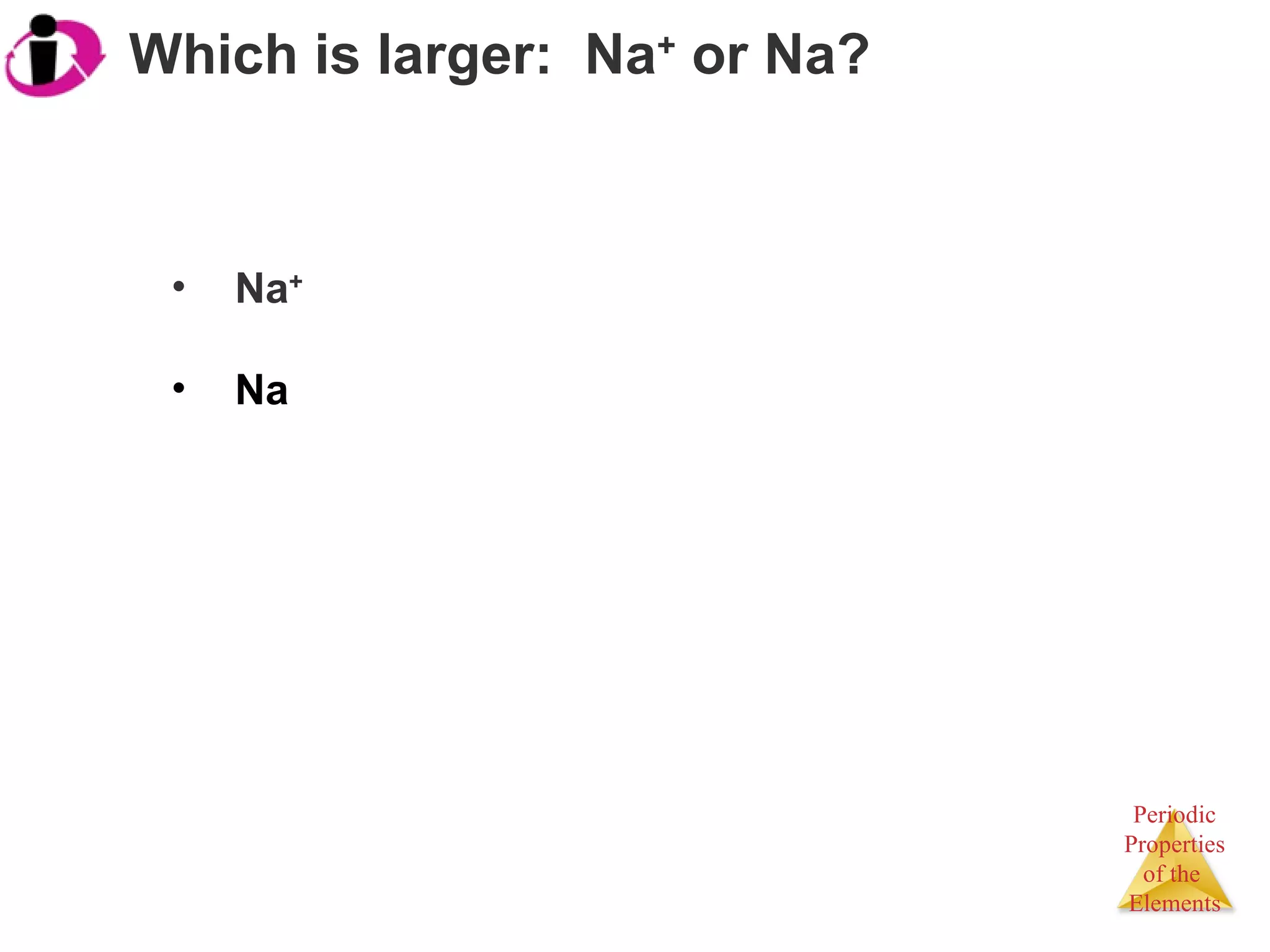






















![They have the same electron configuration: [Ar]3d 3 .](https://image.slidesharecdn.com/7ap-091021075052-phpapp02/75/APChem-Chapter-7-Lecture-Periodic-Trends-60-2048.jpg)