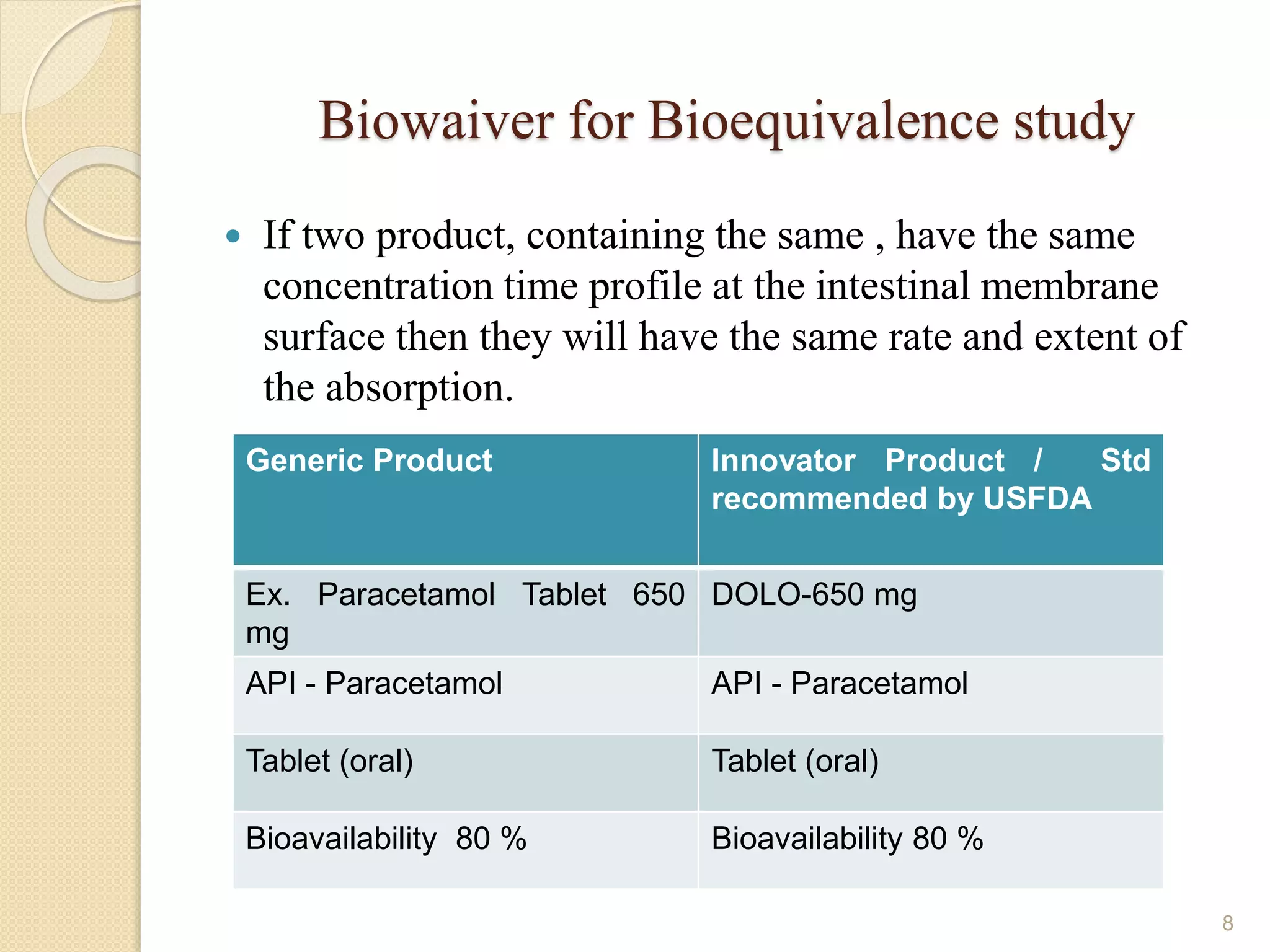
The document discusses the Biopharmaceutics Classification System (BCS), which categorizes drug substances based on solubility and permeability, enhancing understanding of oral drug absorption. It covers the concept of biowaivers, allowing regulatory approval without in vivo testing under certain conditions, and outlines techniques to improve drug bioavailability, including solubility enhancement and alternative routes of administration. The significance of biowaivers is emphasized as they can save time and money in drug development while still ensuring equivalent therapeutic outcomes.