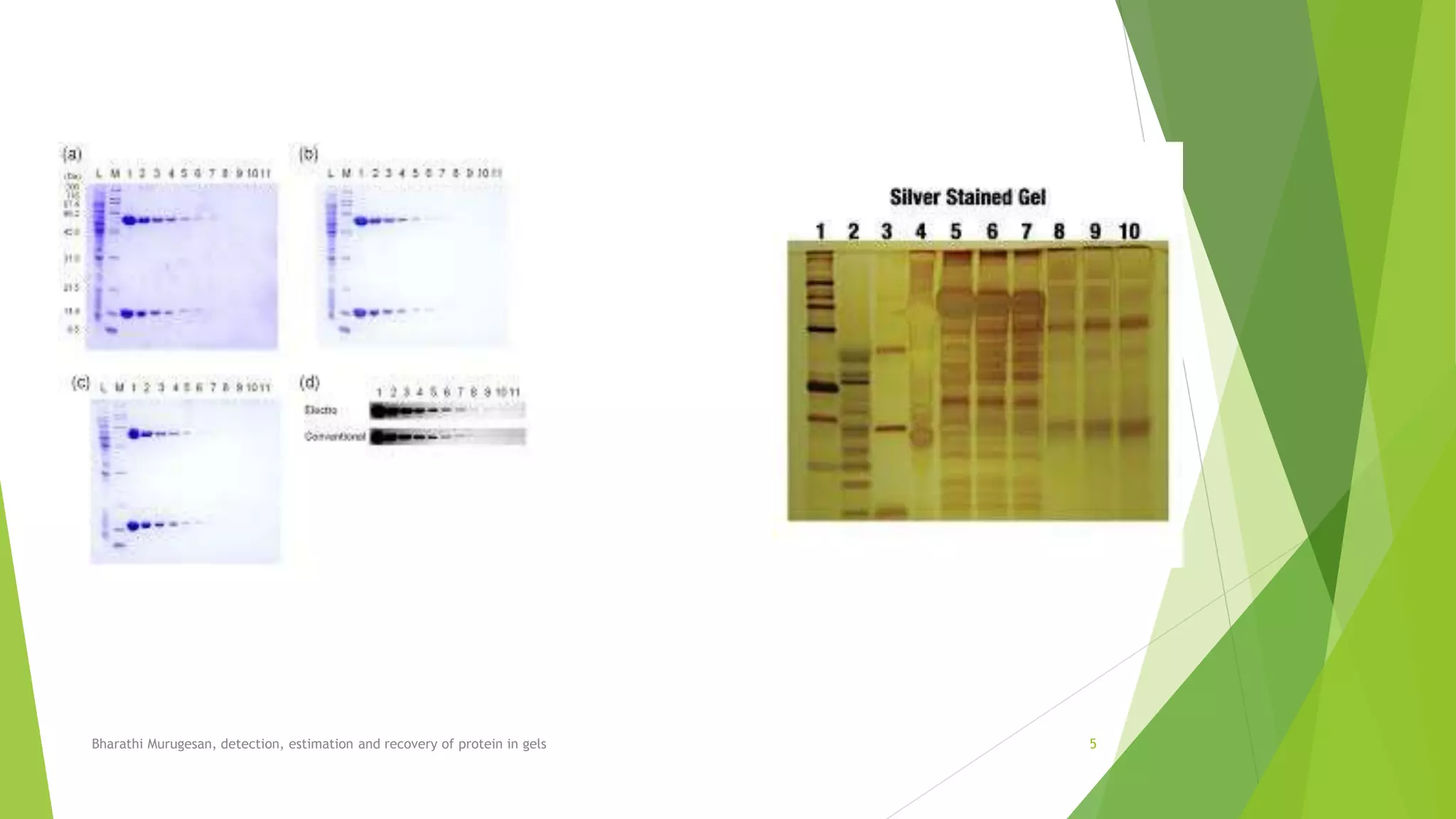
The document discusses methods for detecting, estimating, and recovering proteins in gels. It describes how Coomassie Brilliant Blue R-250 is commonly used to visualize proteins separated via gel electrophoresis. Detection can be improved to 1 ng using silver staining. Protein quantification is achieved through densitometry, while gel documentation systems provide digital analysis. Recovered proteins can be electroeluted from excised gel pieces and identified via mass spectrometry.