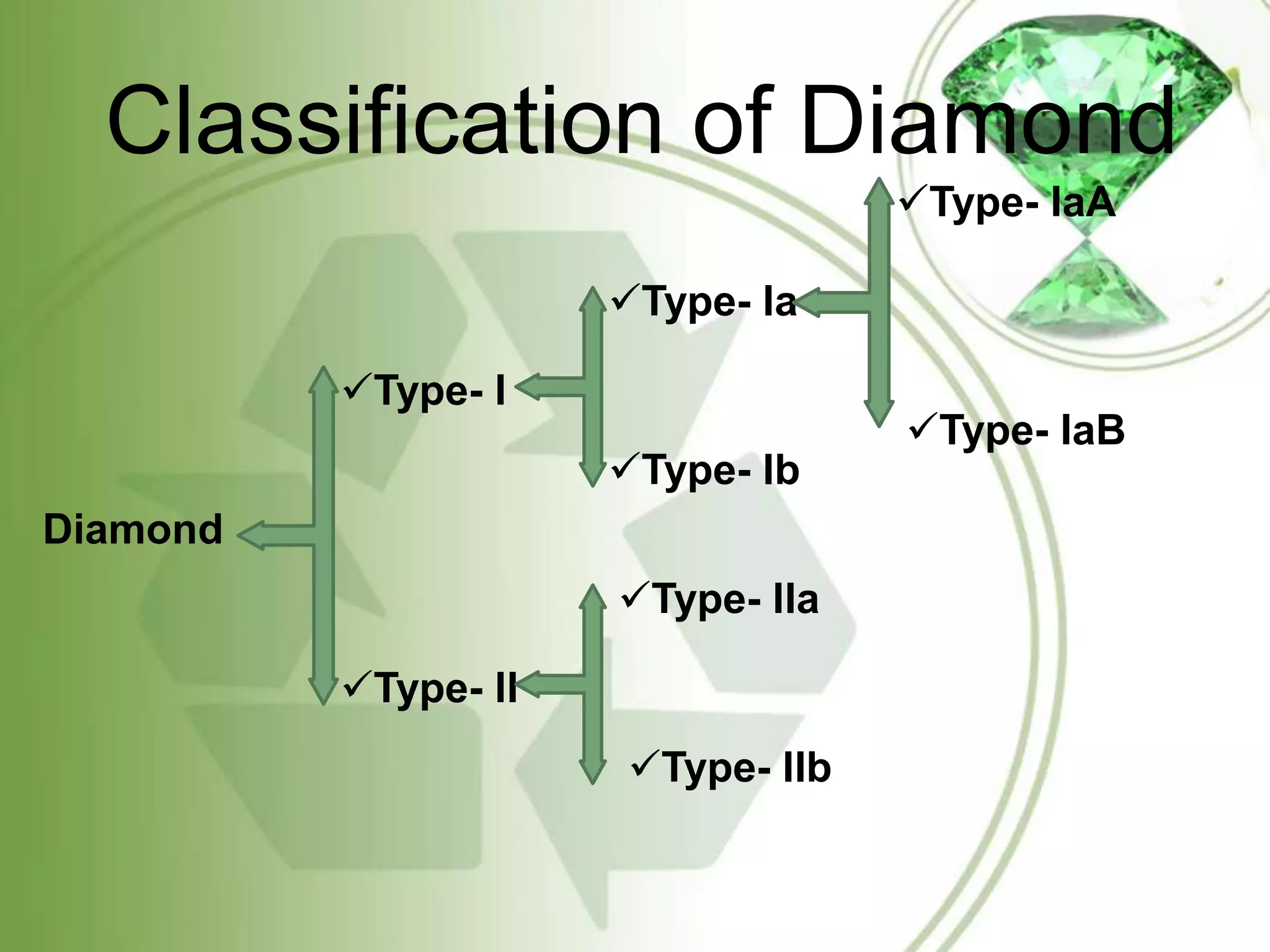
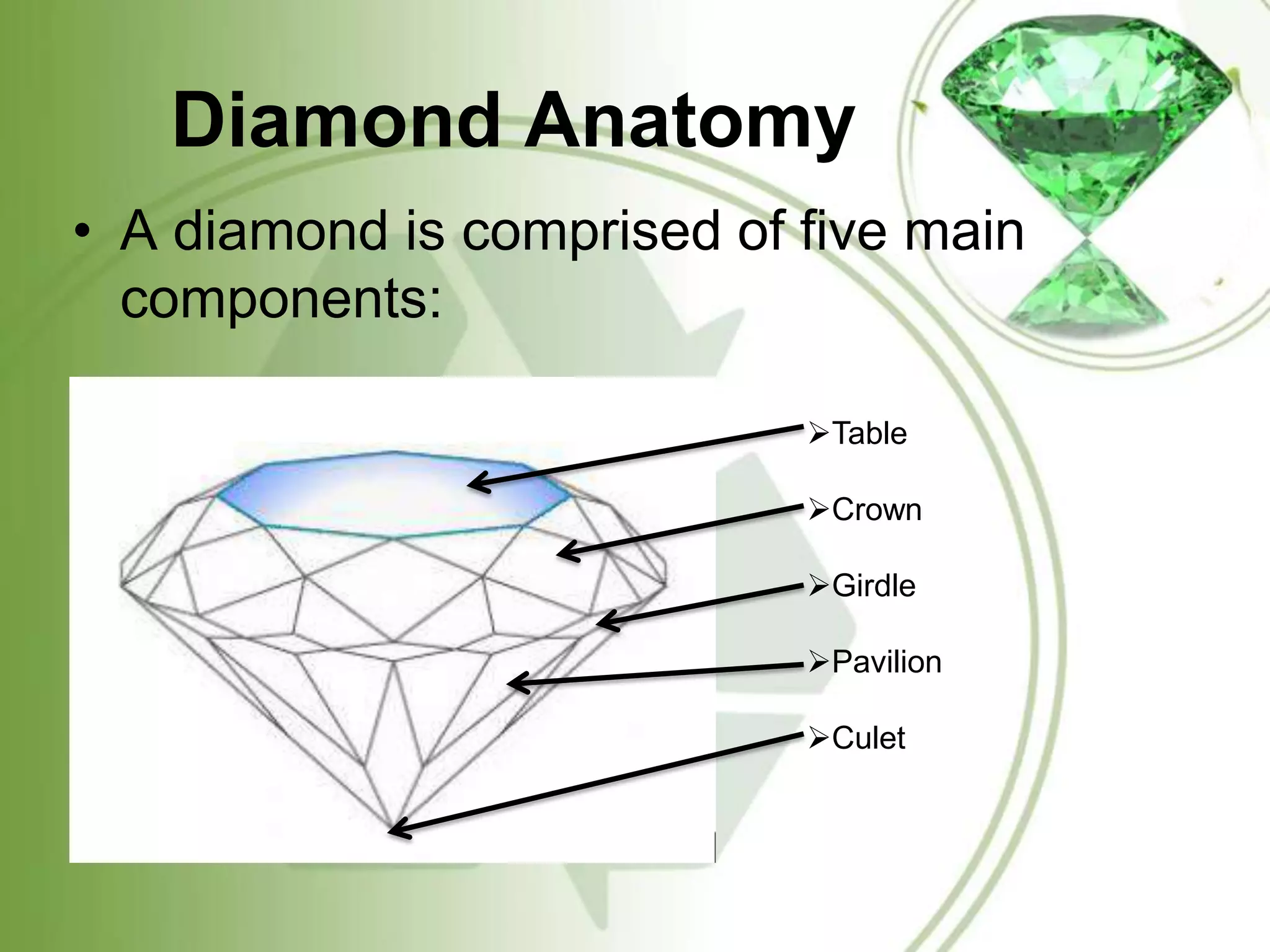
The document summarizes information about diamonds, including their classification, physical and optical properties, formation, and global distribution. Diamonds form 100 miles below the earth's surface under high pressure and temperature. The four main processes that bring diamonds to the surface are deep source eruptions, subduction zone diamonds, asteroid impacts, and diamonds formed in space. India has diamond deposits in states like Madhya Pradesh. The major sources of diamonds are kimberlite, lamproite and eclogite rocks.