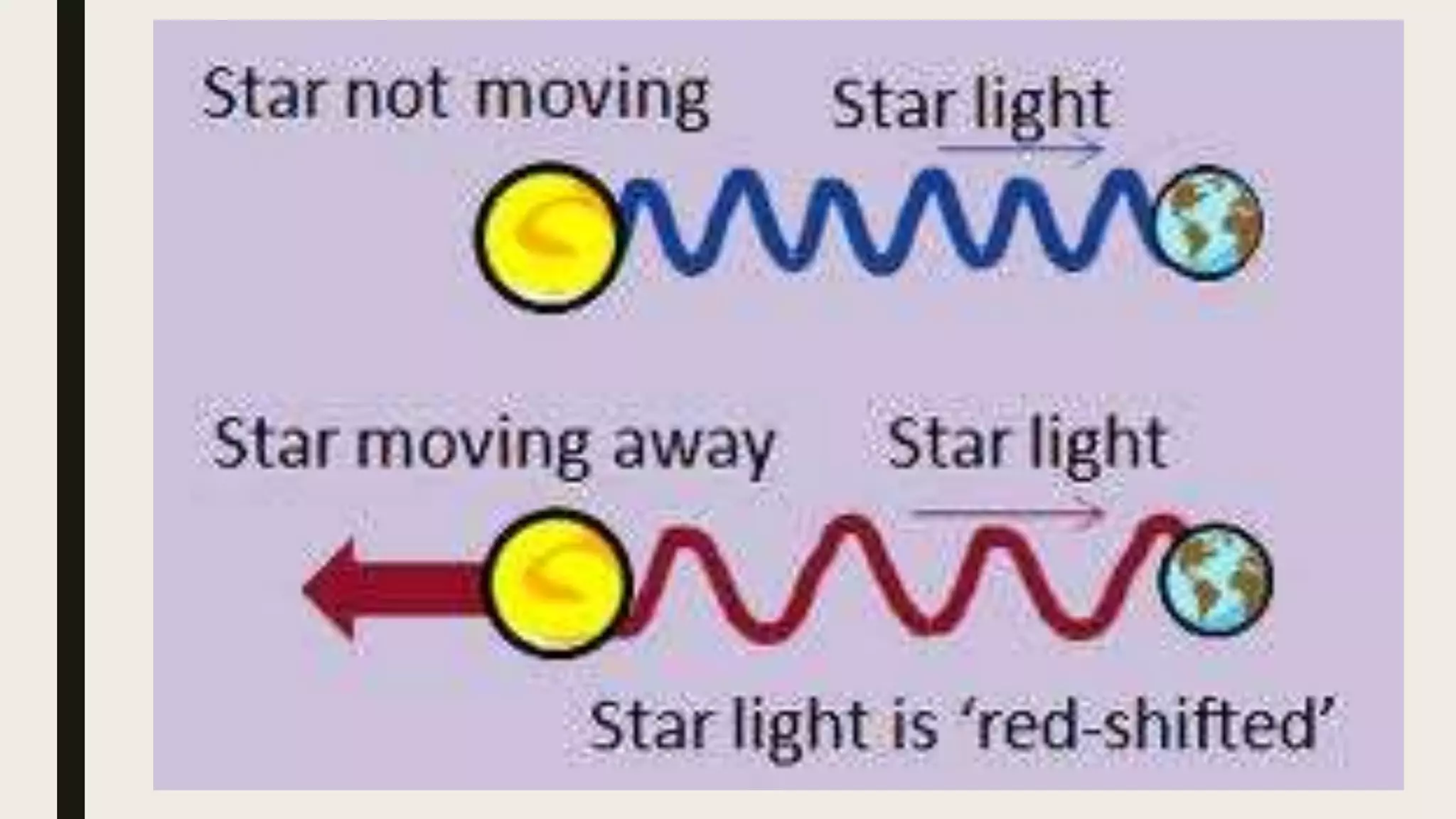
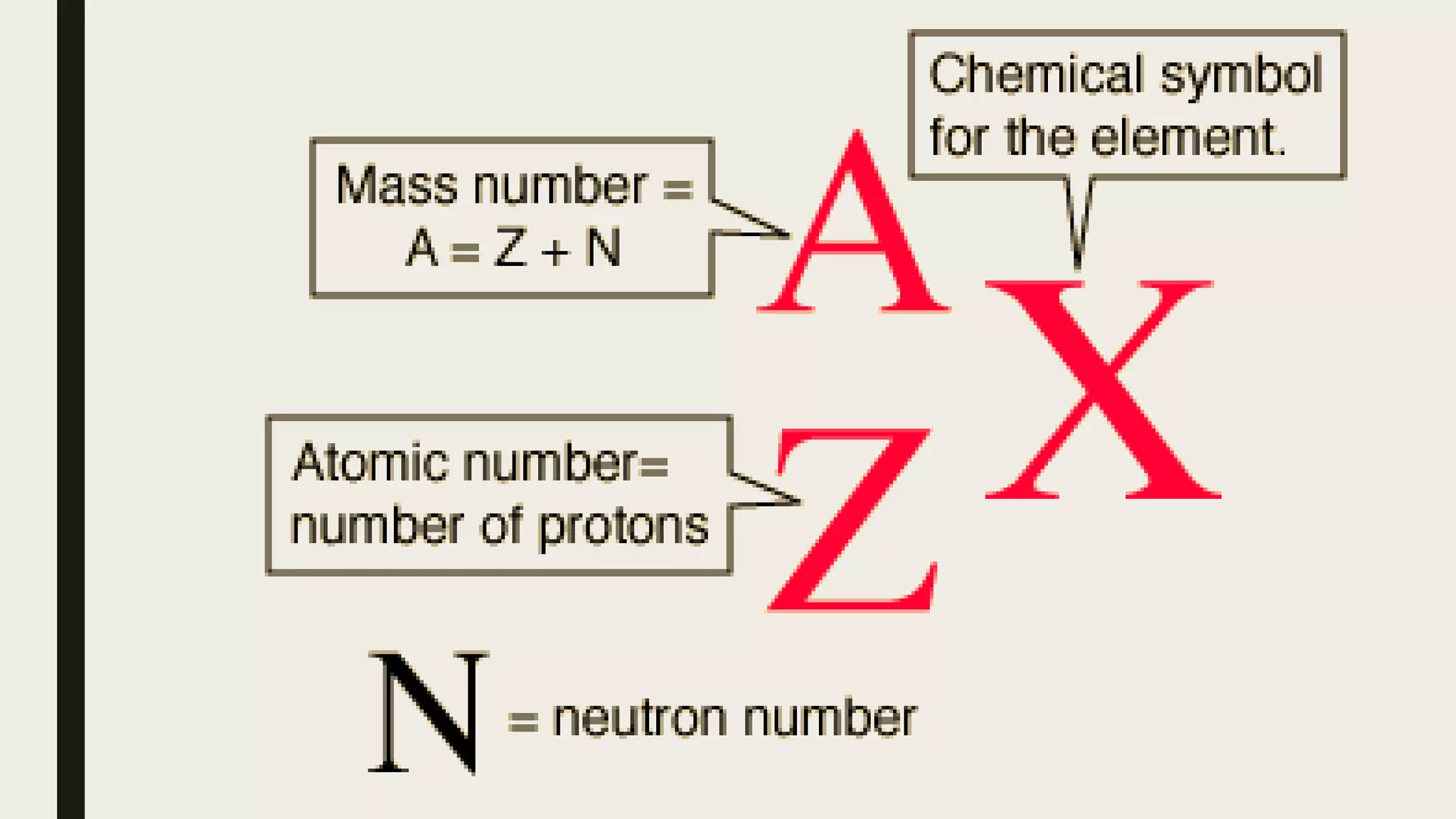
The document outlines a lesson on the Big Bang theory and the formation of light elements, detailing learning objectives related to elemental formation and distribution in the universe. It discusses key concepts such as nucleosynthesis, the role of stellar evolution, and provides various definitions and terms related to physics and chemistry. Additionally, it includes an activity to illustrate the expansion of the universe using balloons and stickers.