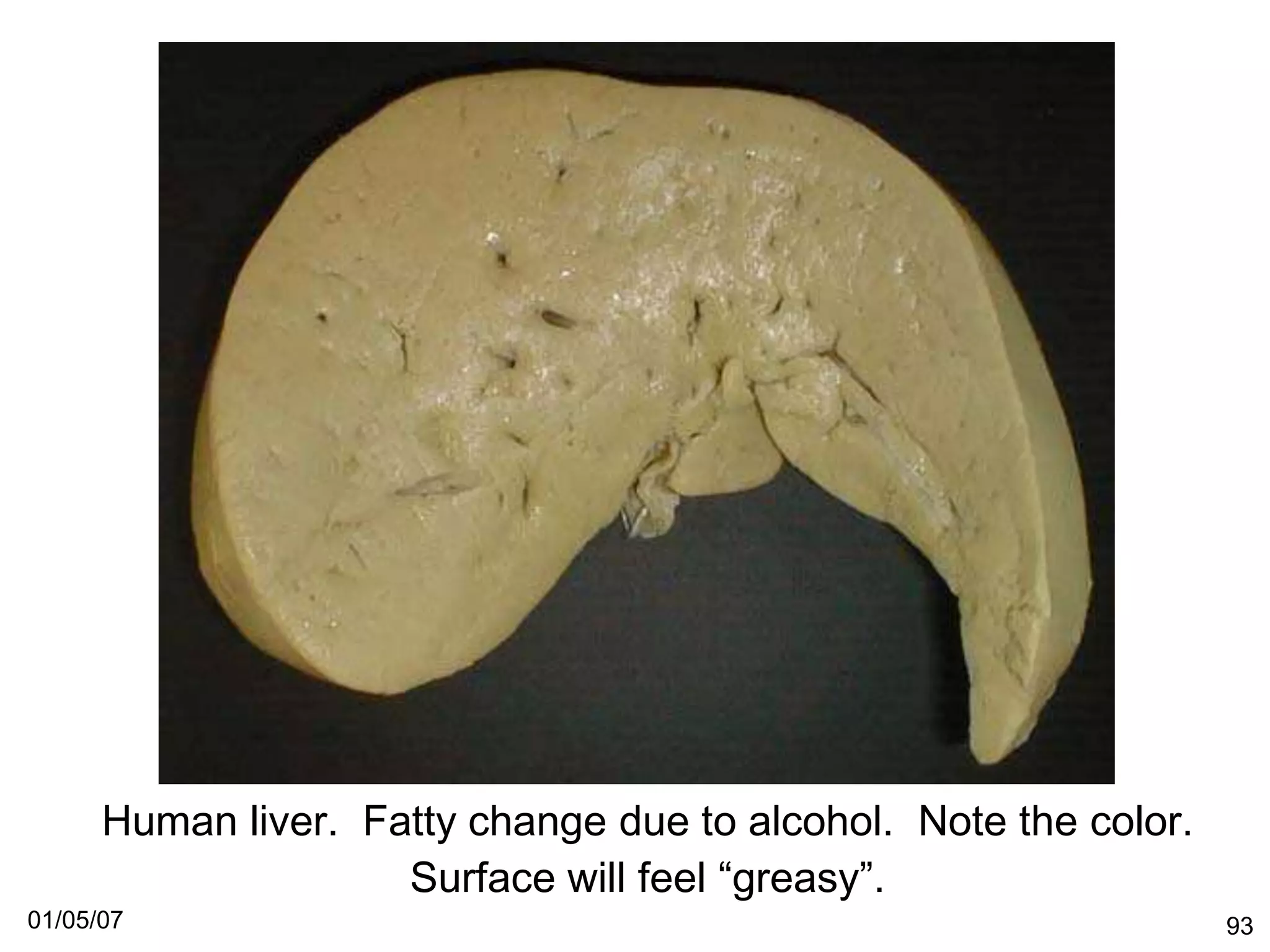
The document provides an introduction to the anatomy and physiology of the liver and rodent stomach. It covers the basic structure of the liver including the hepatic circulation, liver lobule, and liver acinus. It describes the key cell types in the liver including hepatocytes, Kupffer cells, pit cells, and Ito cells. It also discusses the liver's role in bile formation and excretion of bilirubin. Finally, it outlines the sections to be covered in the document, including responses of the liver to toxic injury and interpretation of rodent hepatic and stomach tumor data.







































































































































































































![Blood Enzymes that Reflect Hepatocellular Damage:
Alanine Aminotransferase (ALT [SGPT]).
• Primarily found in hepatocytes.
• Normally present in the serum in low concentrations and
released in high amounts with hepatocellular damage.
• Looking for a 2-3 times increase for biological
significance.
• Level is an indicator of hepatocellular membrane
damage rather than hepatocellular necrosis. Serum level
of ALT is poorly correlated with the degree of liver cell
damage.
• Usually not increased in purely cholestatic disease.
01/05/07 Dr R B Cope 204](https://image.slidesharecdn.com/liver-nicnas-nov-2012-130125012814-phpapp02/75/Liver-nicnas-nov-2012-204-2048.jpg)
![Blood Enzymes that Reflect Hepatocellular Damage:
Aspartate Aminotransferase (AST [SGOT]).
• Primarily found in hepatocytes, cardiac muscle, skeletal
muscle, kidneys, brain, pancreas, lung, leukocytes and
erythrocytes i.e. increased AST in the absence of an
increased ALT suggests another source other than liver.
• Looking for a 2-3 times increase for biological significance
• Other features are similar to ALT.
• Level of AST in some species, e.g. horse, is of no
meaningful value.
01/05/07 Dr R B Cope 205](https://image.slidesharecdn.com/liver-nicnas-nov-2012-130125012814-phpapp02/75/Liver-nicnas-nov-2012-205-2048.jpg)





















































































































