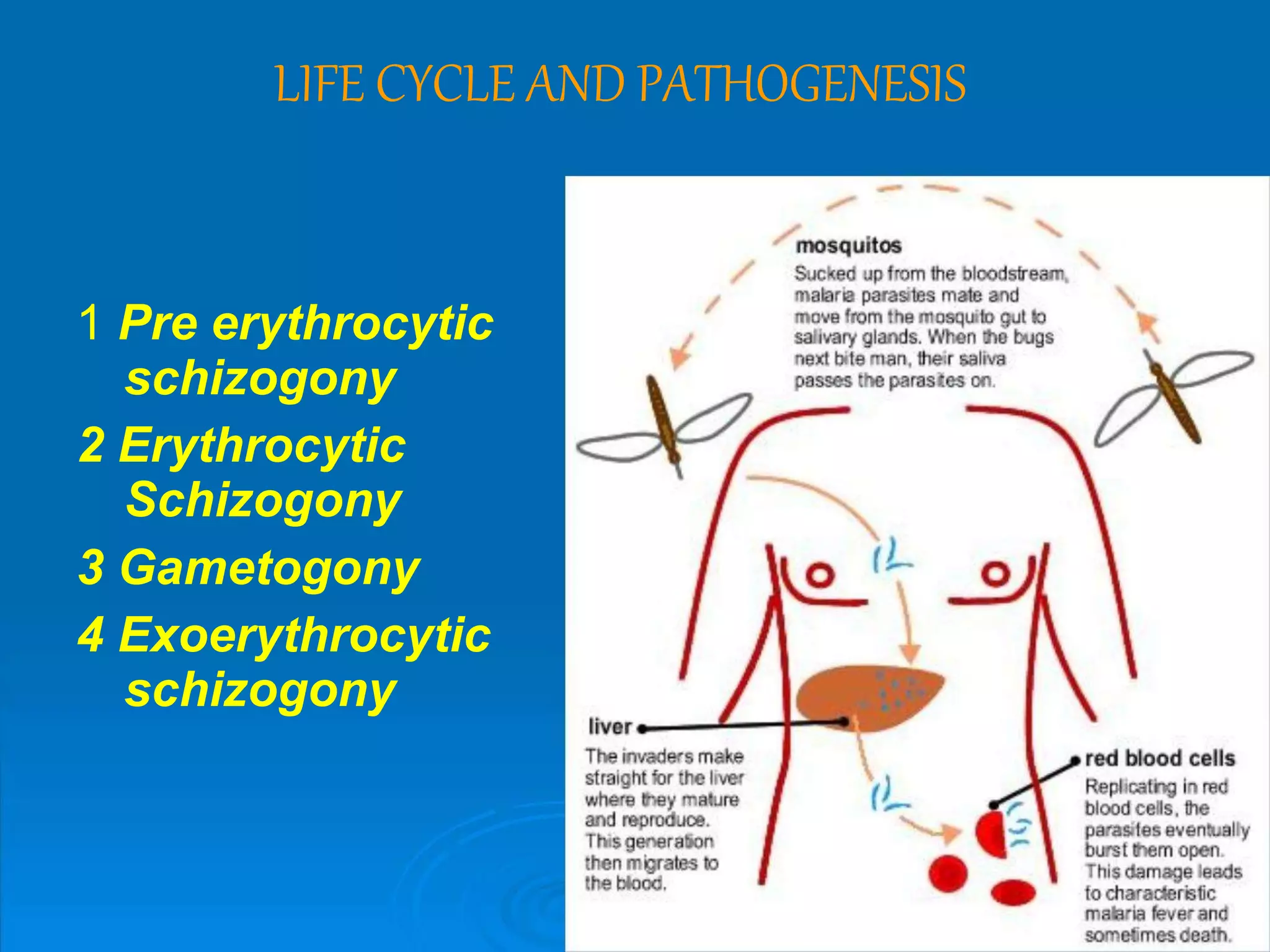
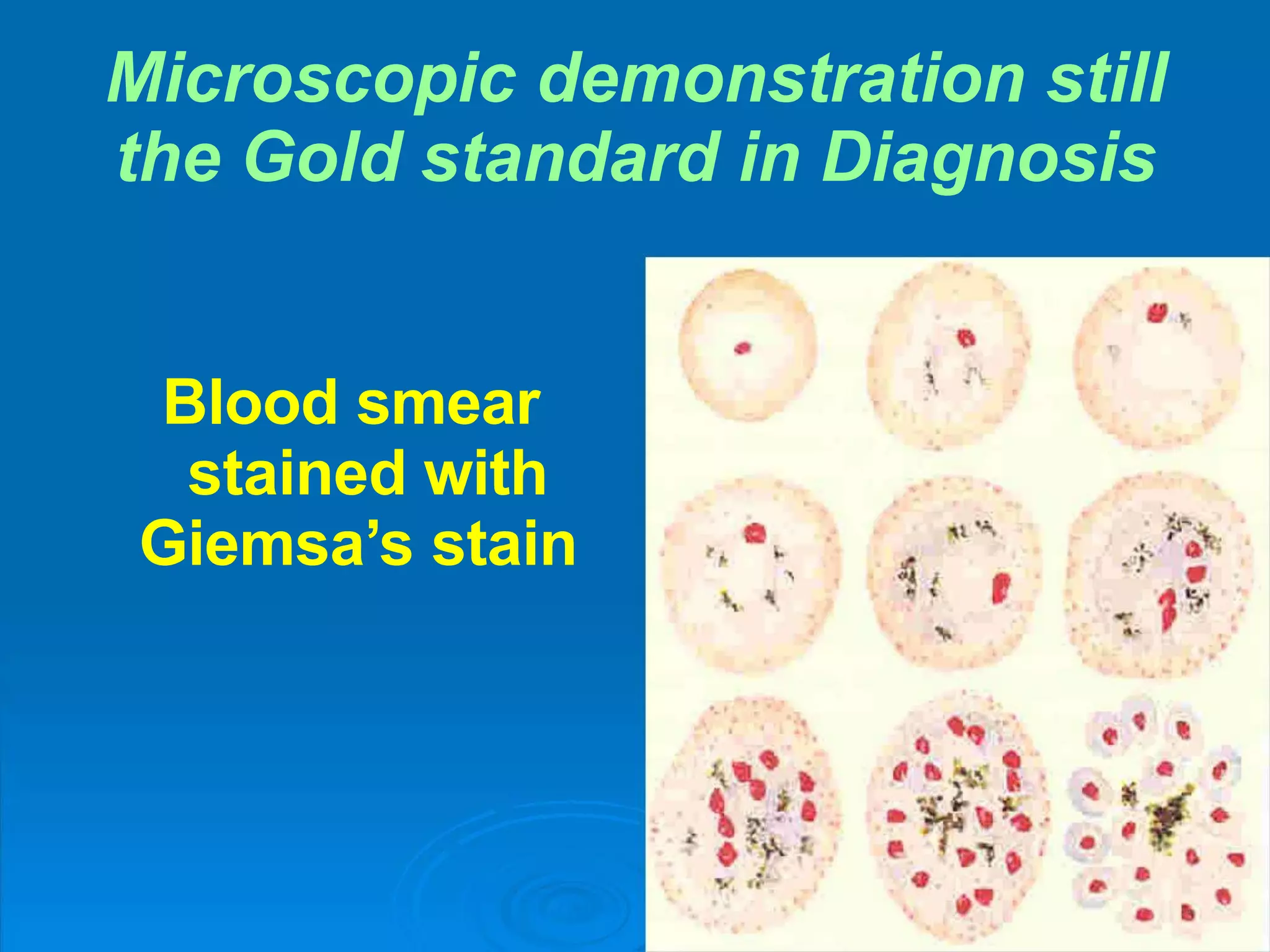
Malaria is a life-threatening disease caused by Plasmodium parasites transmitted via mosquito bites. It remains a major global health issue, infecting hundreds of millions annually and killing over 500,000. The document discusses the definition, history, epidemiology, life cycle and clinical presentation of malaria, focusing on the different Plasmodium species involved, their transmission and symptoms. It also covers treatment, prevention, and highlights those most at risk.