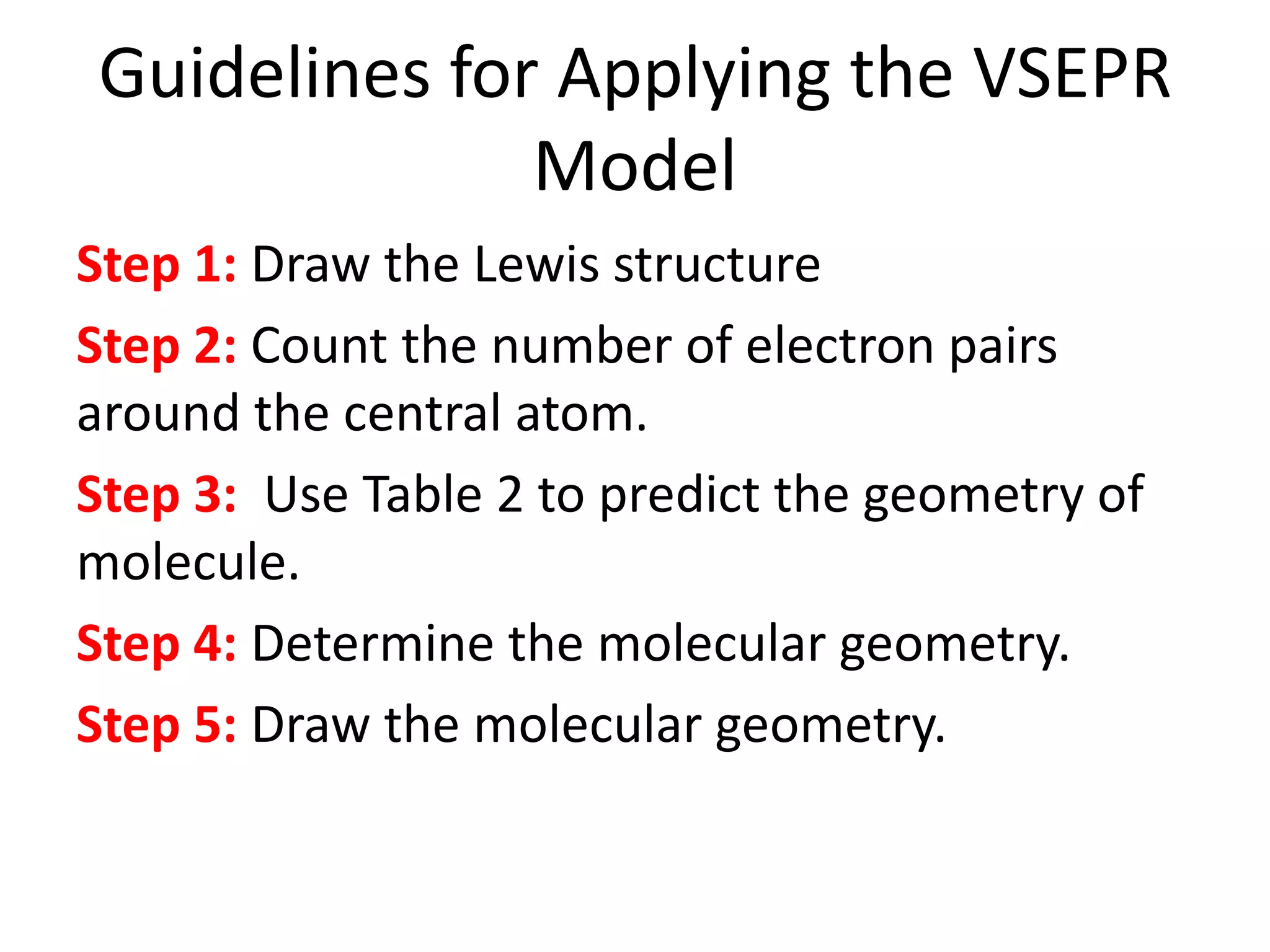
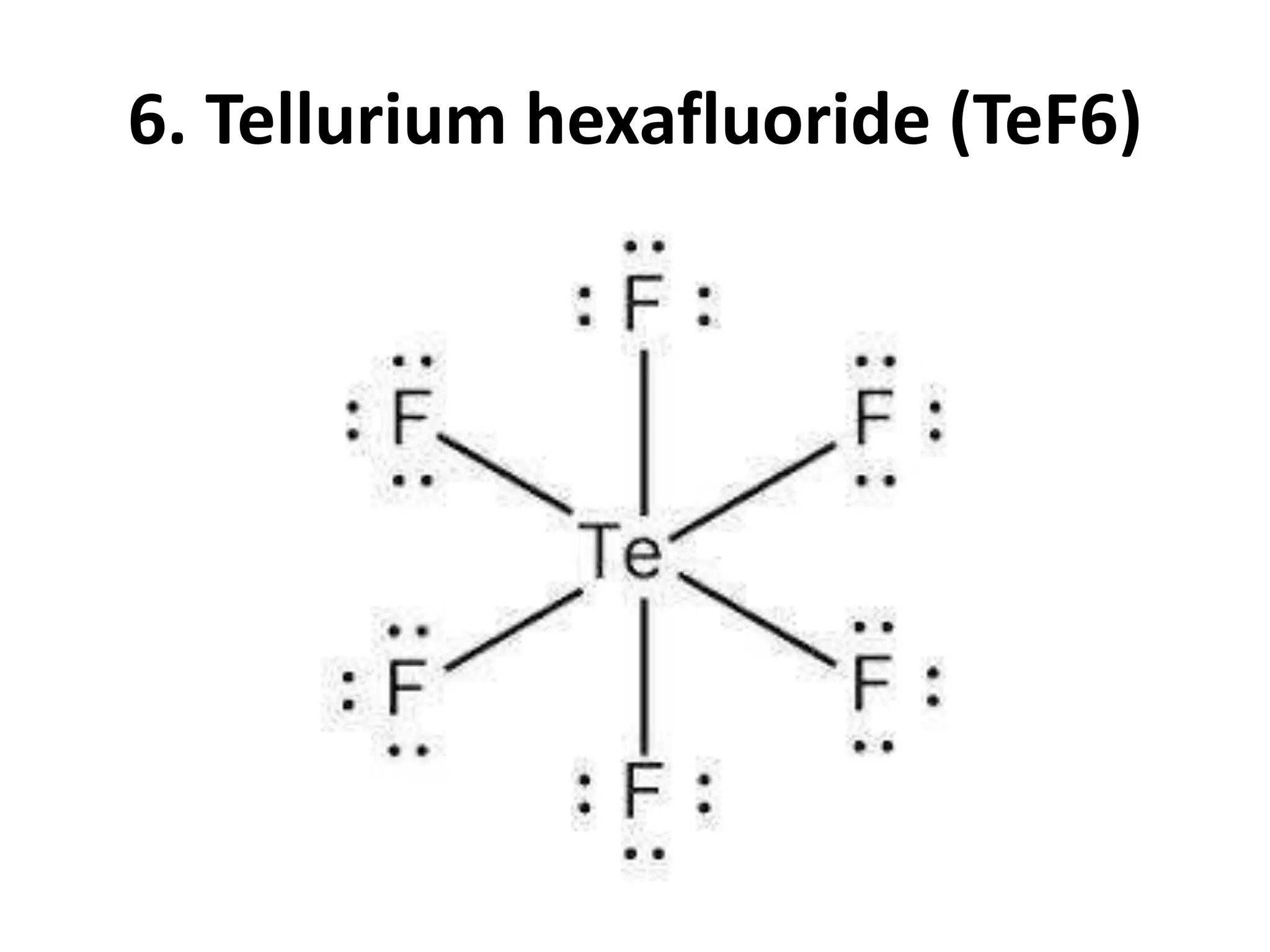
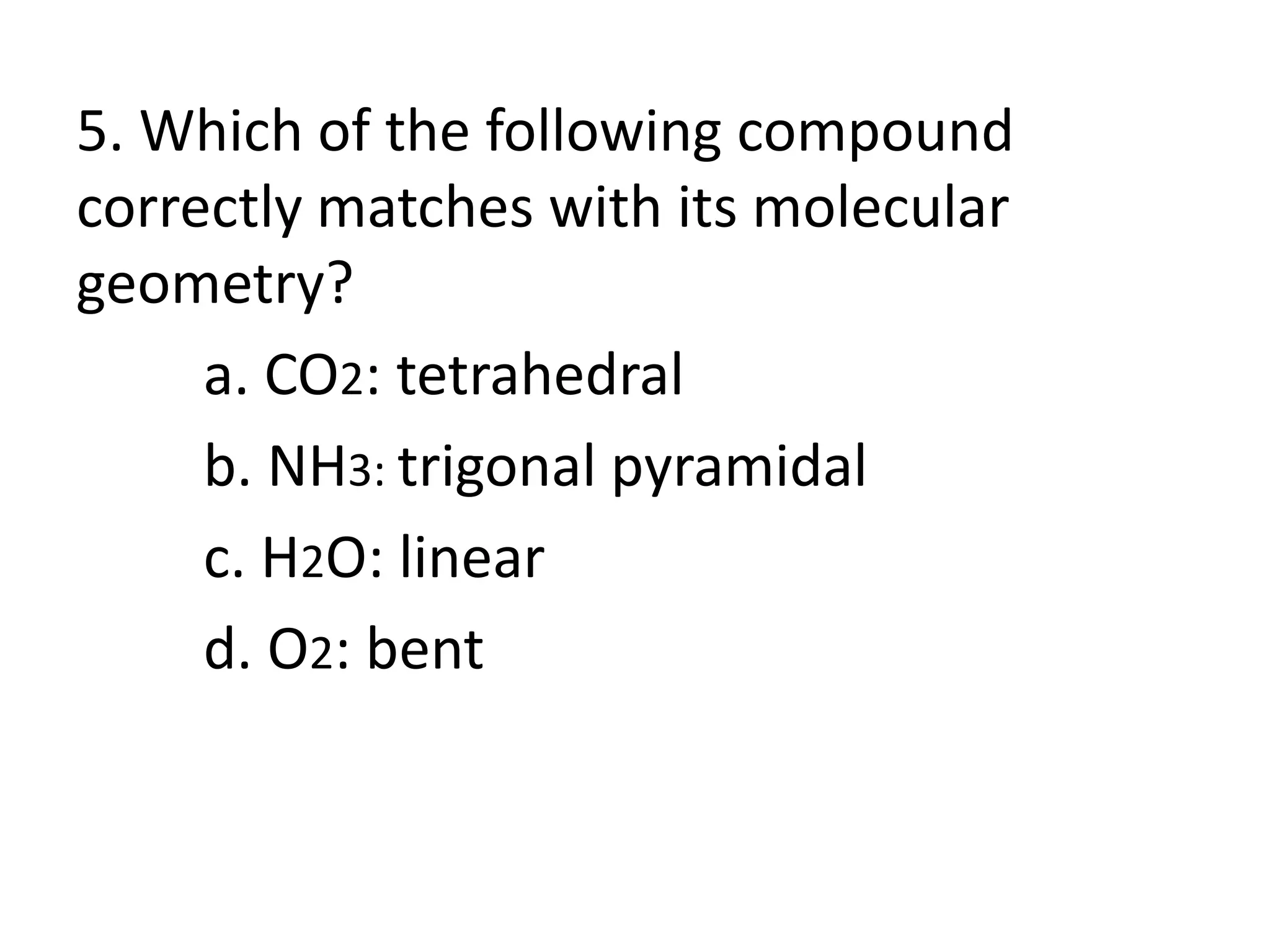
This document provides information on predicting molecular polarity using valence shell electron pair repulsion (VSEPR) theory. It begins by explaining the key concepts of VSEPR theory, such as electron pair-electron pair repulsion and the effects of bond pairs and lone pairs. Examples are given of applying VSEPR theory to determine the molecular geometry and polarity of molecules like H2O, NH3, and SO2. The document emphasizes that molecular geometry is crucial for distinguishing between polar and nonpolar molecules. It concludes by summarizing that symmetric molecular shapes tend to be nonpolar, while asymmetric shapes are usually polar.