Nernst Distribution Law, Statement. Distribution constant,
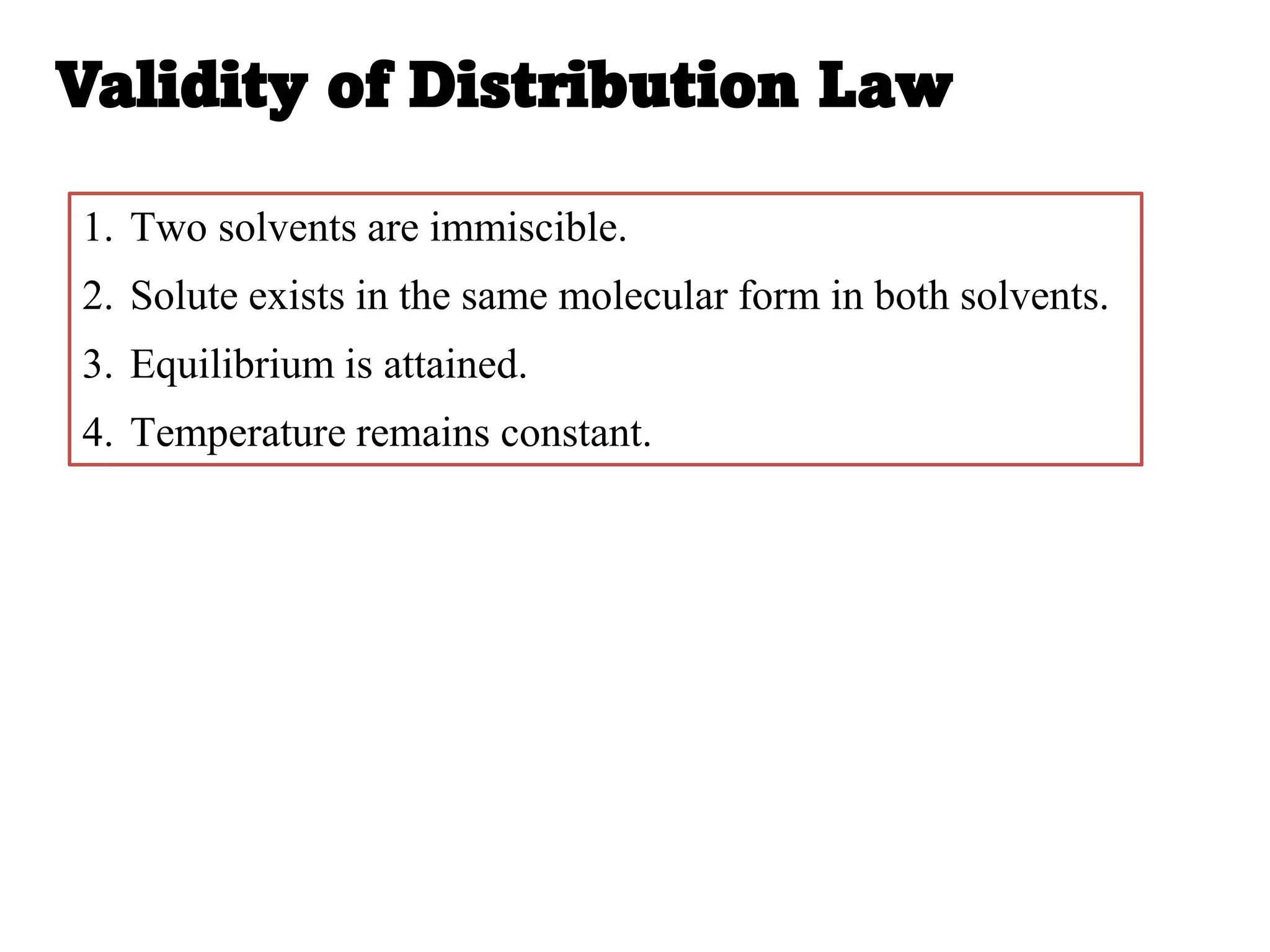
factors affecting distribution constant, validity of distribution law,
modification of distribution law when molecules undergo a)
association b) dissociation. Application of the distribution law