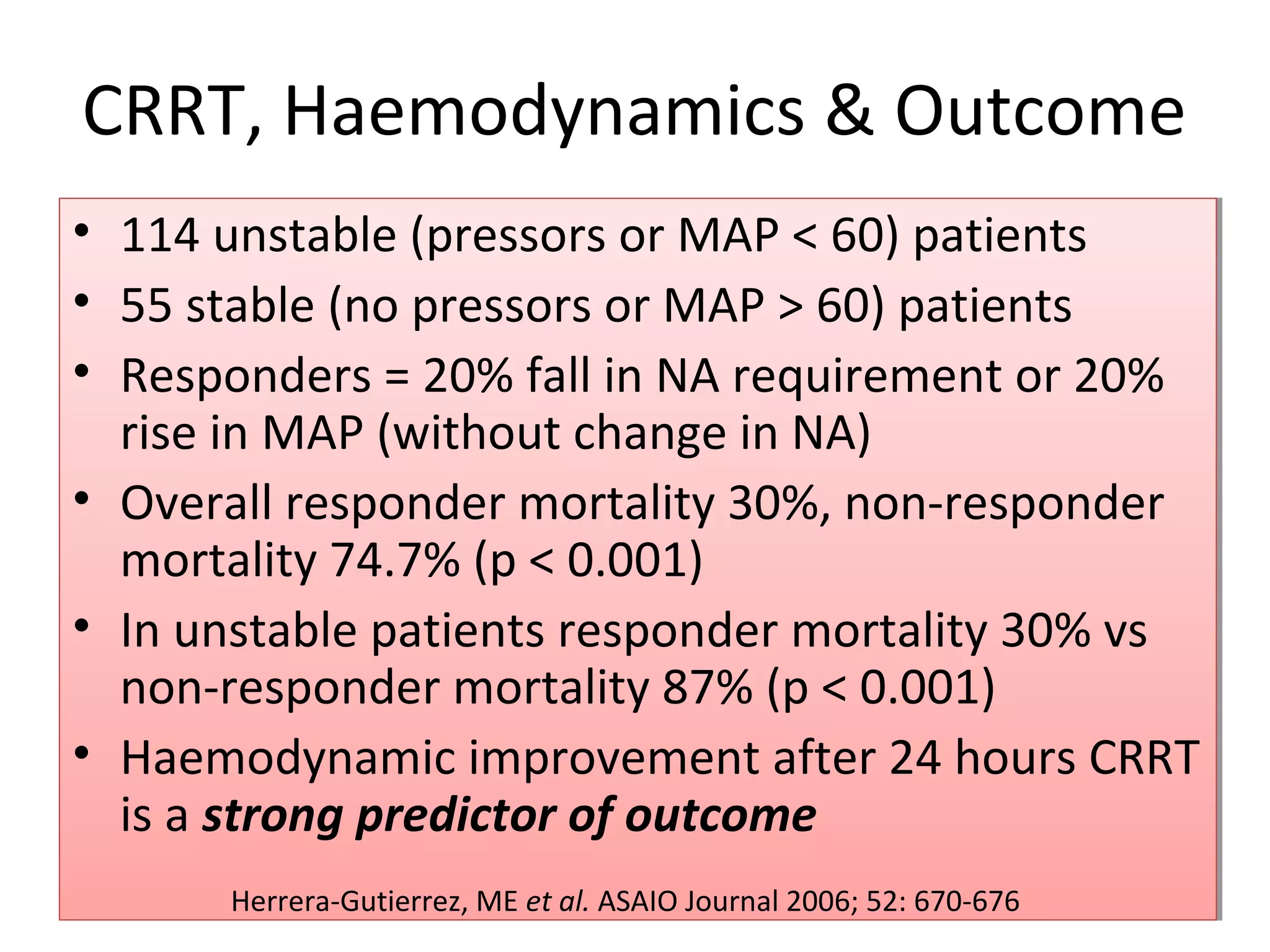
This document discusses renal replacement therapies for acute kidney injury in critical care. It begins by outlining some open questions about optimal therapy use. It then reviews classification systems for AKI severity and evidence that increased severity is associated with higher mortality. The document discusses evidence for relationships between higher therapy dose and better outcomes for intermittent hemodialysis and continuous venovenous hemofiltration. While no definitive evidence establishes the superiority of any one therapy, higher therapy doses are generally associated with better patient outcomes. The document outlines various renal replacement therapy options and their pros and cons.