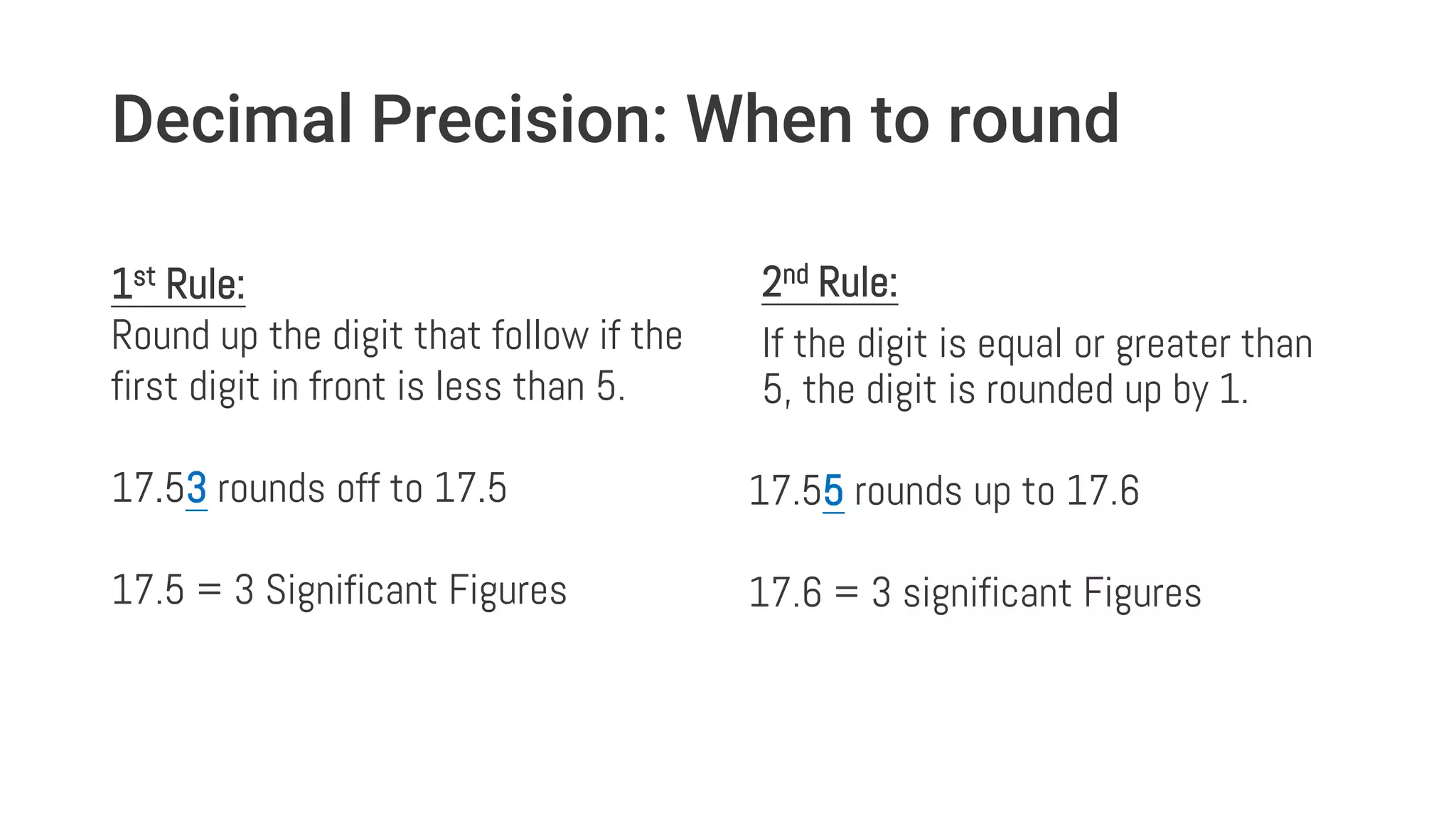
This document discusses the concept of significant figures and how to determine the number of significant figures in measurements and calculations. It defines significant figures as the "important digits" that indicate the precision of a measurement. Rules are provided for determining significant figures depending on leading or trailing zeros and whether the number is read from left to right or right to left. Examples demonstrate applying these rules and how to round final answers in calculations like addition, subtraction, multiplication and division based on the least precise measurement used. The key takeaway is that significant figures convey precision and final answers should not be more precise than the least precise input.